University of Arkansas at Little Rock
Policy Name: Compressed Gas Cylinders (CGC)
Policy Number: LR 701.6
Effective Date: October 6, 2009
Revised Dates: November 3, 2023
Most Recent Review Date: November 3, 2023
Policy
Statement
All compressed gas cylinders used on the UALR campus will comply with the policy detailed in this document.
Purpose
Depending on the particular gas, there is a potential for simultaneous exposure to any of the following types of hazards:
- Decompression
- Flammability and explosion
- Asphyxiation
- Toxicity
- Cryohazard
- Physical hazard (e.g., weight)
No employee shall use any compressed gas cylinder without training in the safe use of these cylinders.
Who Needs to Know
Everyone who uses and works around compressed gas cylinders.
Responsibilities
Managers and Supervisors
- Responsible for ensuring that all requirements listed in the written program for CGC are met.
- Responsible for ensuring new and existing employees are familiar with the CGC program as applicable to their job duties.
- With the assistance of Environmental Health and Safety (EHS), are responsible for identifying CGC hazards.
- Responsible for arranging for required training of university employees in the use of CGCs.
University Employees
- Employees whose duties involve working with CGC are required to comply with the rules of operations and accepted safety practices outlined within this written program.
Environmental Health and Safety Manager
- Responsible for conducting periodic visits to locations where CGC are used. The purpose of these visits is to inspect equipment and to observe employees’ procedures while working with CGC.
- General oversight of this program.
Compressed Gas Cylinder Policy
Identification. No compressed gas cylinder shall be accepted for use that does not legibly identify its contents by name. Color coding is not a reliable means of identification. Cylinder colors vary with the supplier, and labels on caps have little value, as caps are interchangeable. If the labeling on a cylinder becomes unclear or an attached tag is defaced to the point the contents cannot be identified, the cylinder should be marked “contents unknown” and returned directly to the manufacturer.
Caps. To protect the valve during transportation, the cover cap should be screwed on hand and remain on until the cylinder is in place and ready for use. Remove the valve cap only after the cylinder has been safely installed.
Transport. Cylinders that contain compressed gases are primarily shipping containers and should not be subjected to rough handling or abuse. They are built to be as light as possible while remaining safe and durable. Do not drop cylinders or otherwise allow them to strike each other. Such misuse can seriously weaken the cylinder and render it unfit for further use or transform it into a rocket having sufficient thrust to drive it through masonry walls. Cylinders should never be rolled or dragged. When moving large cylinders, they should be strapped to a properly designed wheeled cart or hand truck to ensure stability. Never transport a cylinder with a regulator in place. Always protect the valve during transport by replacing the valve cover. Only one cylinder should be handled at a time. Pulling cylinders by their valve caps, rolling them on their sides or dragging or sliding them can cause damage. Rolling cylinders on their bottom edge (“milk churning”) may be acceptable only for very short distances, as when moving a cylinder into place or onto a cart.
Restraint. Since gas cylinders are tall and narrow, they should be secured in an upright position at all times to prevent tipping. Never lay any cylinders, especially those containing flammable gases, on their sides. Cylinders may be attached to a bench top, individually to the wall, placed in a holding cage, or have a non-slip base attached. Cylinders should be affixed with a bracket to a permanent building fixture such as a bench or wall during use. Brackets that can be screwed into the mounting surface are preferred over clamp-type brackets. It is recommended that cylinders be secured at two locations, at the lower and upper portion of the tank or at a single position where toppling or sliding out from underneath is not probable.
Cylinders containing flammable gases such as hydrogen shall not be stored in close proximity to open flames, areas where electrical sparks are generated, or where other sources of ignition may be present. An open flame shall never be used to detect leaks of flammable gases. All cylinders containing flammable gases should be stored in a well-ventilated area. Store oxidizing gases at least 20 feet away from fuel gases or other combustible materials, or separate them with an approved firewall. Check the reactivity information and storage requirement sections of the MSDS for details about which materials are incompatible with a particular compressed gas.
Cylinders should be placed with the valve accessible at all times. The main cylinder valve should be closed as soon as it is no longer necessary that it be open (i.e., it should never be left open when the equipment is unattended or not operating). This is necessary not only for safety when the cylinder is under pressure, but also to prevent the corrosion and contamination resulting from diffusion of air and moisture into the cylinder.
If compressed gas cylinders are stored outside, use a well-drained, securely fenced area. Keep them on a level, raised concrete pad or non-combustible rack. To prevent excessive pressure buildup, never expose cylinders to temperatures above 52°C (125°F). Some rupture devices will release at approximately 65°C. Do not subject them to temperatures below -29°C (-20°F), unless they are designed for this. Cylinders that become frozen to a surface can be freed by using warm water (less than 52°C). Never apply direct heat to a cylinder.
Discharge compressed gases safely using devices (i.e., pressure regulators) approved for the particular gas. Standard cylinder-valve outlet connections have been devised by the Compressed Gas Association (CGA) to prevent mixing of incompatible gases. The outlet threads used vary in diameter; some are internal, some are external; some are right-handed, some are left-handed. In general, right-handed threads are used for non-fuel and water-pumped gases, while left-handed threads are used for fuel and oil-pump gases. To minimize undesirable connections, only CGA standard combinations of valves and fittings should be used in compressed gas installations; the assembly of miscellaneous parts should be avoided.
The following table, reprinted from an article entitled “Gas Cylinder Safety, Part II – Setup and Use” in LCGC North America magazine, Volume 20, Number 7, July 2002 lists CGA designations for gas cylinders commonly used in the laboratory. These CGA fitting designations can be found inscribed on the inlet nut of the regulator.
Table II: Compressed Gas Association fitting designations for common GC gas cylinders.
| Gas | Fitting Designation |
|---|---|
| Helium | CGA-580 |
| Hydrogen | CGA-350 |
| Air, synthetic | CGA-590 |
| Argon | CGA-580 |
| Nitrogen | CGA-580 |
The threads on cylinder valves, regulators and other fittings should be examined to ensure they correspond and are undamaged. Never force connections or use homemade adapters. Do not lubricate any cylinder valves, fittings, or regulator threads, or apply jointing compounds and tape. Use only lubricants and sealants recommended by the gas supplier. After the regulator is attached, the cylinder valve should be opened just enough to indicate pressure on the regulator gauge (no more than one full turn) and all the connections checked with a soap solution for leaks. Never use oil or grease on the regulator of a cylinder valve. Carefully check all cylinder-to-equipment connections before use and periodically during use, to be sure they are tight, clean, in good condition and not leaking. Carefully open all valves, slowly, pointed away from you and others, using the proper tools. Close all valves when cylinders are not in use. Never tamper with safety-relief devices in cylinders, valves or equipment. Grit, insects, dirt, oil, or dirty water can cause gas leaks if they get into the cylinder valve or gas connection. Use a lint-free tissue to remove any dirt or rust. (Never open the high-pressure cylinder valve to clean the fitting seat.) Never open a damaged valve. Contact your gas supplier for advice. If you suspect the regulator is leaking, return it to the vendor for repair. Never attempt to repair a regulator, valve, or safety device yourself.
Tubing. Instrument connecting tubing and fittings must also be rated to the gas used. They must be able to withstand the highest possible pressure to which they could be subjected in the event of pressure regulator failure.
General. Read the MSDS and labels for all of the materials you work with. Know all of the hazards (i.e., fire/explosion, health, chemical reactivity, corrosivity, and pressure) of the materials you work with. Always use safety glasses (preferably a face shield) when handling and using compressed gases, especially when connecting and disconnecting compressed gas regulators and lines.
Handle “empty” cylinders safely as you would a full cylinder. Leave a slight positive pressure (approximately 200 psi) in them to prevent contamination of the interior of the cylinder. When the cylinder needs to be removed or is empty, close all cylinder valves, bleed remaining gas from the system (when it is safe to do so), disassemble equipment properly, and replace cylinder protection caps. Mark cylinders “empty” or “MT,” and store them separately from full cylinders until they are picked up by the supplier.
In the event of any emergency involving a compressed gas cylinder, evacuate the entire building immediately by sounding the fire alarm and follow the procedures for general evacuation as outlined in the laboratory’s Chemical Hygiene Plan. The Occupational Safety and Health Administration (OSHA) regulations governing the use of compressed gases in the workplace (29 CFR, Parts 1910.101 through 1910.105) can be found at the following URL: https://www.osha.gov/SLTC/compressedgasequipment/index.html. Information on types of high-pressure fittings that connect to gas cylinders can be found on the Compressed Gas Association (CGA) website: http://www.cganet.com.
Documentation of Training
Reporting Requirements
- Representatives of Environmental Health and Safety are authorized to document unsafe acts, advise employee’s supervisor, and stop unsafe work from continuing.
- Supervisors may counsel or take other corrective action to address failure to adhere to guidelines of this program.
- Employees shall report any safety concerns to their supervisor and/or Environmental Health and Safety.
Training Requirements and Competency Assessment
Under no circumstances will any university employee work with compressed gas cylinders until he or she has attended training in the safe use of these devices. This includes all new employees regardless of previous experience. The training program includes but is not limited to:
- Information of the different types of compressed gas cylinders.
- How hazards are to be controlled – engineering controls, administrative controls (use of caps, restraints, regulators), personal protective equipment (PPE).
- Policies and procedures for working around compressed gas cylinders.
- The written Compressed Gas Cylinder Manual.
- Enforcement of rules and corrective actions to be taken for noncompliance.
Documentation of Training will be kept at:
Environmental Health and Safety Office
UALR Facilities Management
2801 S. University Avenue
Little Rock, AR 72204
Records Should Include the Following Information:
- Date of training.
- Employee printed name and signature.
- The agenda or a list of the topics covered.
Program Evaluation
The EHS Committee will evaluate the written CGC Program annually, and periodic audits will be conducted by EHS. All updates, changes, and additions will be documented and will be kept with the written program. When evaluating the program, the following items will be reviewed to measure the program’s overall effectiveness.
- Accident/incident reports
- Medical records
- Management/employee compliance
- Recommendations
- Inspections
- Training records
- Administrative/Engineering controls
Website Address for This Program
Contacts, Telephone, Email Address
Vince Rodgers, EHS Manager, 501-916-6351, varodgers@ualr.edu
David Millay, Facilities Management Director, 501-569-8897, dlmillay@ualr.edu
Keith Hudson, Director GIT, 501-569-8211, mkhudson@ualr.edu
Related Information
Compressed Gas Cylinders Authority and References:
OSHA 29 CFR 1910.101 (Compressed Gases – General Requirements)
OSHA 29 CFR 1910.102 (acetylene)
OSHA 29 CFR 1910.103 (hydrogen)
Compressed Gas Association (CGA) (safety publications)
National Fire Protection Agency (NFPA) (compressed gas)
Definitions
Decompression. Sudden decompression can propel a cylinder with enough force to penetrate building walls. When the gas pressure is released rapidly through an opening the size of the valve stem, a cylinder can reach velocities of close to 66 mph. Since the gases are contained in heavy, highly pressurized metal containers, the large amount of potential energy resulting from compression of the gas makes the cylinder a potential rocket or fragmentation bomb capable of causing catastrophic property damage, personal injury, and death if they are not handled properly.
Asphyxiation. Liquefied gases may expand as much as 1000-fold in volume when vaporized. When the content of a large cylinder is vented very rapidly, symptoms of oxygen deficiency or even asphyxiation may occur through atmospheric displacement.
Flammability or Explosion. If the gas is flammable, flash points lower than room temperature present a danger of fire or explosion. Even breathing air can pose a hazard as a combustion accelerator. Adequate ventilation must always be used to prevent the build-up of vapors of flammable gases such as hydrogen, methane, and acetylene. Adequate ventilation is also required when using gases such as nitrogen, helium, or hydrogen. In these cases, oxygen can be condensed out of the atmosphere creating a potential for explosive conditions.
Toxicity. Generally, the gases encountered in this lab are not considered toxic; that is to say, once a victim has been removed from an area of exposure, the immediate effects of the gas exposure (e.g., dizziness or difficulty breathing) will diminish rapidly. However, the MSDS must always be consulted for toxicological information prior to working with any gas.
Cryohazard. Liquefied gases such as carbon dioxide and nitrogen can cause immediate frost burns on exposed skin. Even very brief contact with a cryogenic liquid is capable of causing tissue damage similar to that of thermal burns. Prolonged contact may result in blood clots that have potentially serious consequences. In addition, surfaces cooled by cryogenic liquids can cause severe damage to the skin. Gloves and eye protection (preferably a face shield) should be worn at all times when handling cryogenic liquids.
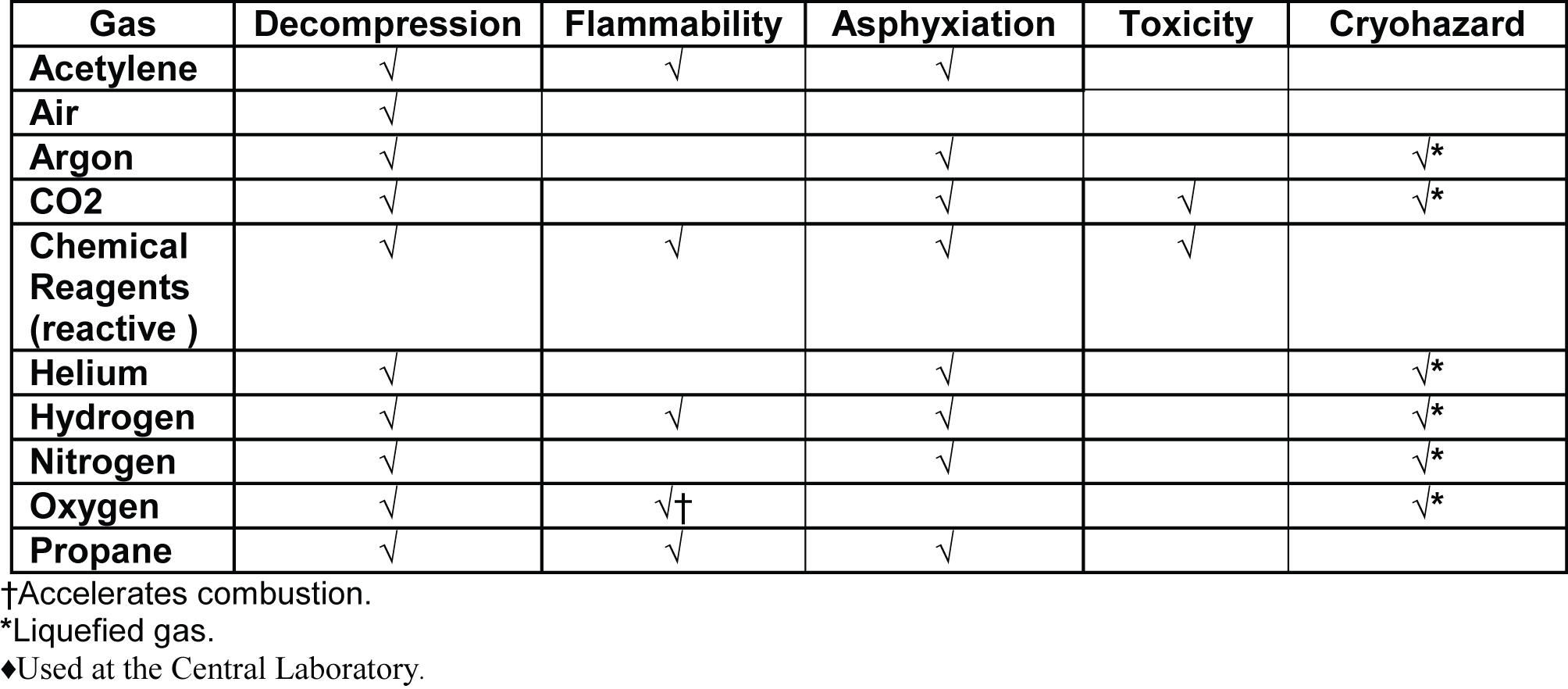
Physical Hazard. An empty cylinder can weigh up to 200 lbs. or more depending on the size of the cylinder and the density of the gas contained within. No one should attempt to lift a cylinder that weighs more than 50 lbs. For this reason, all cylinders used in this lab pose a heavy lifting hazard. The following table, reprinted from an article entitled “Gas Cylinder Safety, Part I – Hazards and Precautions” in LCGC North America magazine, Volume 20, Number 6, June 2002, categorizes these hazards per gas type:
Table I: Hazard classes for commonly used GC gases and other gases found in the laboratory.
Source: Environmental Health and Safety
Status: Decommissioned
Approved By: Environmental Health and Safety Committee, Oct. 6, 2009
Originator:
Custodian: EHS Committee